Equine Joint Supplementation: Does It Really Work?
Dietary supplements are pharmaceutical alternatives that claim to have physiological benefits. These products have become increasingly popular in veterinary medicine to treat or manage various disorders resulting from age or stress placed on performance horses. In 2012, an equine industry survey reported:
- 77 percent of horse owners provide at least one supplement to the diet of their horse(s).
- 35 percent for performance enhancement
- 34 percent for preventing/treating joint disorders
- 63 percent of respondents incorporate joint supplements into their horse’s diet.
It is not surprising that joint supplementation is very common among performance horse owners and trainers, considering lameness and osteoarthritis are two of the most common equine ailments. Osteoarthritis is a disease process associated with alterations in the structure and function of synovial joints, resulting from a loss of balance between the synthesis and degradation of essential macromolecules. It is most commonly considered an age-related issue; however, some research has suggested that repeated heavy loading or injury may also induce the onset of the disease. The typical symptoms are pain, stiffness, and limitation of motion, eventually resulting in difficulties performing daily activities and a lower quality of life.
There are many products currently marketed as joint supplements for human and equine use, and the most common “active ingredient” found in them is glucosamine, which is synthesized naturally in the body and present in high quantities in joint cartilage, synovial fluid, and vertebral discs. Synovial joints allow for movement and are covered with cartilage and lubricated by synovial fluid. Glucosamine is important in forming various components of articular cartilage and synovial fluid and is shown to protect chondrocytes, which are cells responsible for maintaining the composition and organization of the cartilage and synovial fluid matrix. The main idea behind the use of joint supplements is to supply “building blocks” for articular cartilage, which may help delay, stabilize, or even repair osteoarthritis-related changes to the joint.
The Risks of Supplement Use
Despite the popularity of supplements in both human and veterinary medicine, there are various issues surrounding their use. Dietary supplements, in general, are not FDA-approved or regulated. FDA approval of a product means that data on the product’s effects have been reviewed and determined to provide benefits that outweigh potential risks. However, under the Dietary Supplement Health and Education Act, dietary supplement firms do not need FDA approval before marketing their products, making it the company’s responsibility to ensure their products are safe and claims are valid.
Since the DSHEA does not demand the same rigorous requirements for quality manufacturing for supplements as it does for pharmaceuticals, there is potential for dietary supplements to provide lower-quality materials or not meet labeled quantities. In other words, just because you see a supplement product on a store shelf does not mean it is safe or effective, because there are no requirements for manufacturers to conform to quality control or quality assurance practices.
Unfortunately, this lack of regulation can lead to supplement contamination. In 1998, California investigators discovered that nearly one-third of 260 imported herbal products included unlisted drugs or contained potentially hazardous levels of lead, mercury, or arsenic. Another case in 2009 showed a vitamin/mineral supplement prepared by a compounding pharmacy contained toxic levels of selenium, resulting in the death of 21 horses. In 2017, several horses were dismissed from competition due to positive drug tests, which were eventually traced back to a gastric nutritional supplement that contained unlisted levels of ractopamine, an International Federation for Equestrian Sports banned substance.
Cases of contamination are not as rare as one would hope. From 2013 to 2016, a total of 221 equine deaths, injuries, and positive drug tests from contaminated feed were reported. Additionally, several review studies have indicated that the most common contaminants of equine supplements are heavy metals, pesticides, Dimethylsulfoxide (DMSO), morphine, and caffeine.
Another concern with these products is that some have been shown to falsely advertise ingredients. Oke and colleagues investigated 23 glucosamine-containing equine oral joint supplements and found that 13 of the 23 products contained less of the active ingredient than advertised on the label, with three containing less than 30 percent and one containing no glucosamine at all. Table 1 outlines the details of the glucosamine label claim. These results are similar to those reported by Russell et al. in 2002, who found that only two out of 14 over-the-counter glucosamine-containing products for humans contained the advertised amount of the active ingredients.
|
Label claim (mg glucosamine/50mg product |
Measured amount (mean ± SD) |
% measured glucosamine |
|---|---|---|
|
1.70 |
0.5 ± 0.0 |
29.4 |
|
4.39 |
0.5 ± 0.0 |
11.4 |
|
29.60 |
2.9 ± 0.0 |
9.8 |
|
11.00 |
0.0 ± 0.0 |
0.0 |
Also mentioned in the study by Oke et al. was the inconsistency of dosing recommendations between the products (varying from 1,800 to 10,000 mg/day). Although there is no official standard recommended glucosamine dosage for horses, some studies have suggested a dosage of around 10,000 mg/day for a mature horse. Based on this dosage, only five of the 23 products investigated recommended close to this dosage, with the mean recommendations being about half. Additionally, based on the glucosamine levels measured, a 10,000 mg dose would only be achieved by two of the 23 products, with several products not meeting their own daily recommended dose.
Implications of Peer-Reviewed Studies
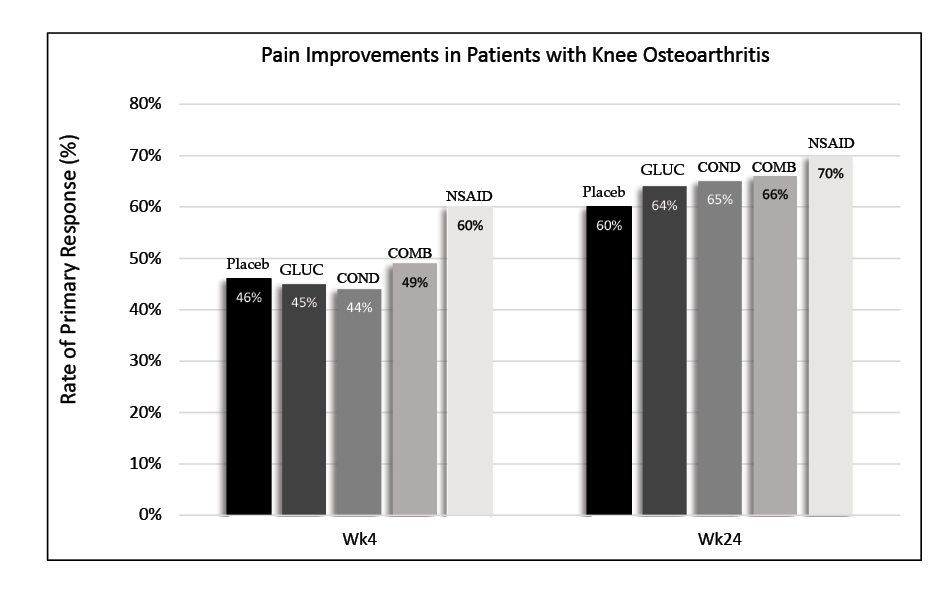
Another issue surrounding the use of joint supplements is the lack of peer-reviewed studies supporting oral joint supplement effectiveness in both humans and horses. Moreover, the studies that do exist are sometimes problematic. Some studies on isolated joint cells have proposed that glucosamine may enhance the production of certain molecules in the synovial joint and protect chondrocytes. However, results have been inconsistent or conflicting between studies. While glucosamine may have some anti-arthritic properties, the question remains whether exogenous glucosamine can even penetrate the joint and reach the chondrocytes. According to the Glucosamine/Chondroitin Intervention Trial in 2006, pain improvement between the non-steroidal anti-inflammatory drugs (NSAIDs), joint supplement, and placebo groups was not significantly different.
Research on oral joint supplementation in horses has also been inconclusive. One study demonstrated that oral glucosamine had around 5 percent bioavailability. This means that a large portion of orally administered glucosamine was rapidly eliminated from the body. The researchers concluded that oral glucosamine dosing resulted in serum and synovial fluid concentrations too low to modify joint cell activity and that joint tissue cells used the extra glucosamine slowly or not at all.
Another study in 2014 supplemented a group of aged horses with either a joint supplement mix or a placebo and did not see any improvement in stride length. It should be noted that the study did observe improvements in carpal flexion and fore fetlock extension tests; however, the improvement was seen in both groups (control and treated). The improvement was, therefore, attributed to the increase in exercise in the older horses, not the supplement.
What about studies performed over the past decades that have reported beneficial effects of glucosamine in cases of osteoarthritis? Unfortunately, the validity of many of these studies with positive results has been called into question due to a variety of significant concerns. One concern is the lack of a placebo group. The absence of a placebo group makes any improvements seen in a study subjective, as there is nothing to compare them to.
Many of these studies are also inconsistent with each other, using different forms of glucosamine, such as glucosamine sulfate or glucosamine hydrochloride, or using commercially available products versus compounds in pure form procured from a lab. Finally, one of the biggest issues many reviewers have with some of these studies is apparent bias. Some studies reporting positive results have been sponsored by the same companies that manufacture the supplement. Company sponsorship significantly increases the likelihood of positive results in trials of non-steroidal anti-inflammatory drugs or supplements. Many review articles investigating glucosamine trials also note this trend.
Another form of bias apparent in equine trials is evaluators not being blinded to the treatments. Because osteoarthritis pain improvements are hard to evaluate in horses, many studies rely on observing changes in various ranges of movement as an outcome measure. Several studies of equine oral joint supplements not only lacked a placebo group but also had evaluators who were aware of the treatment being administered. This may result in observational bias.
To Supplement or Not to Supplement?
Horse owners should proceed with caution when deciding whether to use an oral joint supplement for their horse due to the issues discussed above. However, if horse owners decide to use a joint supplement, there are steps they can take to ensure they are using a high-quality, safe product. The ACCLAIM system was developed so practitioners could rapidly evaluate a joint supplement based on information provided on the label to identify and recommend appropriate products:
A represents a recognizable name. Is the product in question manufactured by a recognizable equine company? In general, established companies dedicated to improving the quality and efficacy of joint-health supplements are more likely to produce superior products. Additionally, equine companies will understand the specific dietary sensitivities of horses.
C represents clinical experience. Find companies that support clinical research, have their products tested in clinical trials, and have research published in peer-reviewed journals. These publications should be readily accessible.
C is a reminder to review the contents of the product. All ingredients should be listed on the label, including active ingredients, inactive ingredients, and fillers.
L is a reminder to pay attention to label claims. Note the product claims on the label. If the claims sound too good to be true, they probably are. Identify products with realistic label claims based on scientific study results, rather than testimonials. The FDA does have regulations regarding the types of claims that can be made on a nutritional supplement; however, illegal claims, such as those claiming to treat, cure, or prevent a disease, are abundant.
A represents administration recommendations. Dosing instructions should be accurate and easy to follow. The amount of active ingredient administered per dose per day should be easy to calculate. Some companies may make dose calculations challenging to mislead consumers. Products with clear administration recommendations and recommended dosages based on published clinical trials are more likely to be of higher quality.
I is a reminder to review the identification information. Find products with a lot identification number or some other tracking system. This suggests that the company has some form of pre- and/or post-market surveillance system to ensure product quality is in place. Producing a supplement akin to a pharmaceutical drug shows a long-term investment in their product and company.
M is a reminder to review the manufacturer information, which should be clearly stated on the label, preferably along with contact information or a website for customer support.
Horse ownership can be complicated and, at times, overwhelming. Owning horses is expensive, and reducing unnecessary expenses is a goal for many horse owners. Understand that horses sometimes experience lameness and injuries. Knowing the efficacy of given therapeutic regimens can reduce the costs of ownership by eliminating expensive and ineffective products. The more knowledge you have about the products available, the more enjoyable and worthwhile the horse-owning experience will be.
References
Centers for Disease Control and Prevention. (2020). Osteoarthritis (OA). Centers for Disease Control and Prevention.
Chard, J., & Dieppe, P. (2001). Glucosamine for osteoarthritis: Magic, hype, or confusion? BMJ, 322(7300), 1439–1440.
Clegg, D. O., Reda, D. J., Harris, C. L., Klein, M. A., O’Dell, J. R., Hooper, M. M., Bradley, J. D., Bingham III, C. O., Weisman, M. H., Jackson, C. G., Lane, N. E., Cush, J. J., Moreland, L. W., Schumacher Jr., H. R., Oddis, C. V., Wolfe, F., Molitor, J. A., Yocum, D. E., Schnitzer, T. J., … Williams, H. J. (2006). Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. The New England Journal of Medicine, 354(8), 795–808.
Delafente, J. C. (2000). Glucosamine in the treatment of osteoarthritis. Rheumatic Disease Clinics of North America, 26(1), 1–11.
Gugliotta, G. (2000, March). Health concerns grow over herbal aids. The Washington Post, A1.
Higler, M. H., Brommer, H., L’Ami, J. J., de Grauw, J. C., Nielen, M., van Weeren, P. R., Laverty, S., Barneveld, A., & Back, W. (2014). The effects of three-month oral supplementation with a nutraceutical and exercise on the locomotor pattern of aged horses. Equine Veterinary Journal, 46(5), 611–617.
Jerosch, J. (2011). Effects of glucosamine and chondroitin sulfate on cartilage metabolism in OA: Outlook on other nutrient partners especially omega-3 fatty acids. International Journal of Rheumatology, 2011, 969012–17.
Laverty, S., Sandy, J. D., Celeste, C., Vachon, P., Marier, J., & Plaas, A. H. K. (2005). Synovial fluid levels and serum pharmacokinetics in a large animal model following treatment with oral glucosamine at clinically relevant doses. Arthritis and Rheumatism, 52(1), 181–191.
McAlindon, T. E., LaValley, M. P., Gulin, J. P., & Felson, D. T. (2000). Glucosamine and chondroitin for treatment of osteoarthritis: A systematic quality assessment and meta-analysis. JAMA, 283(11), 1469–1475.
Montague, T. (2017). Contaminated feed and supplements. BioStar.
Oke, S. (2008, May 1). Oral joint supplements for horses. The Horse.
Oke, S., Aghazadeh Habashi, A., Weese, J. S., & Jamali, F. (2006). Evaluation of glucosamine levels in commercial equine oral supplements for joints. Equine Veterinary Journal, 38(1), 93–95.
Oke, S., & McIlwraith, C. (2008). Review of the potential indications and contraindications for equine oral joint health supplements. AAEP Proceedings, 54, 261–267.
Papich, M. G. (2016). Saunders handbook of veterinary drugs (4th ed.). Elsevier.
Rochon, P. A., Gurwitz, J. H., Simms, R. W., Fortin, P. R., Felson, D. T., Minaker, K. L., & Chalmers, T. C. (1994). A study of manufacturer-supported trials of nonsteroidal anti-inflammatory drugs in the treatment of arthritis. Archives of Internal Medicine, 154(2), 157–163.
Russell, A., Aghazadeh-Habashi, A., & Jamali, F. (2002). Active ingredient consistency of commercially available glucosamine sulfate products. The Journal of Rheumatology, 29(11), 2407–2409.
Stowe, C. (2012). Results from 2012 Association for Healthcare Philanthropy equine industry survey.
Thomas, K. (2009). Toxic dose of selenium cited in deaths of polo horses. The New York Times, 158(54660).
United States Equestrian Federation, & US Equestrian Communications Department. (2017, May 9). Cargill acknowledges contamination of feed supplement caused positive test results. US Equestrian.
The information given here is for educational purposes only. References to commercial products, trade names, or suppliers are made with the understanding that no endorsement is implied and that no discrimination against other products or suppliers is intended.
Publication 3646 (POD-06-24)
By Hannah Valigura, Graduate Research Assistant, and Clay Cavinder PhD, Professor and Extension Horse Specialist, Animal and Dairy Sciences.
The Mississippi State University Extension Service is working to ensure all web content is accessible to all users. If you need assistance accessing any of our content, please email the webteam or call 662-325-2262.



